- BeanWealth
- Posts
- Biotech's Sleeper Hit
Biotech's Sleeper Hit
A $17M Shot at Cancer Breakthrough...
Good Evening! 👋
Dear Investor,
Biotech stocks are starting to move again.
After nearly two years of pain, the sector is finally waking up. Capital is flowing back into early-stage drug platforms. Small-cap names are getting volume again. And retail investors are starting to pay attention to a space that’s been ignored for too long.
But while everyone’s chasing what already ran, there’s one stock most people haven’t noticed yet.
GT Biopharma is a U.S.-based immunotherapy company trading under $1. They’ve developed a cancer-fighting platform that aims to deliver the same clinical power as CAR-T therapy, but without the cost, delay, or genetic modification.
It’s early, and the company is still under the radar. But if this technology scales, it could change how we treat cancer altogether.
Let me break it down.
Why GT Biopharma Is Different
Most people have heard of CAR-T therapy. It’s one of the most promising cancer treatments in the world. But it’s also expensive, slow, and complicated. Doctors have to harvest a patient’s cells, send them off to be modified in a lab, then reinfuse them weeks later. That process costs hundreds of thousands of dollars and often requires elite cancer centers to even be available.
GT Biopharma is going after the same cancer targets with a completely different approach.
Instead of reengineering a patient’s immune cells, GT uses what are called natural killer cells, or NK cells. These are part of your body’s first line of defense against cancer. The company’s core platform, called TriKE, is designed to supercharge these NK cells and guide them to attack specific tumors.
TriKE stands for “Tri-specific Killer Engager.” It’s a small molecule with three parts. One binds to the cancer cell. One binds to the NK cell. And the third delivers IL-15, a protein that boosts the NK cell’s ability to multiply and kill.
The result is a therapy that works off the shelf. It doesn’t require any cell harvesting or genetic modification. It can be infused like a regular drug. And it’s flexible enough to be adapted to different types of cancer just by changing the targeting mechanism.
That matters because it’s not just a single drug. It’s a platform. Think of it like a software engine that can be reused and upgraded. Once the system is proven in one cancer type, it opens the door to many more.
The RFK Jr. Reform Tailwind
Biotech doesn’t just move on clinical results. It moves on policy too. And right now, there’s a real shift happening in how people are thinking about drug approvals.
At the center of it is Robert F. Kennedy Jr.
He’s been vocal about changing how the FDA works, especially when it comes to getting experimental therapies to patients faster. His campaign has focused on giving more control to doctors and patients, cutting down the layers of bureaucracy, and putting the spotlight on treatments that traditional systems tend to overlook.
For a company like GT Biopharma, that could change everything.
Their TriKE platform isn’t a one-size-fits-all drug. It’s a flexible, modular system. That kind of innovation sometimes gets stuck in the current approval process. But if a Kennedy administration pushes for faster access and reform, GT could benefit directly from those changes.
Nothing is guaranteed, and this isn’t about politics. It’s about understanding how a shift in Washington could unlock value in platforms that are already built, waiting for the right environment to scale.

A Flexible Pipeline and a Clear Focus
GT Biopharma isn’t spreading itself thin. Instead of chasing dozens of indications at once, the company is methodically building out a pipeline that starts with blood cancers and expands into solid tumors.
The second TriKE candidate in development targets HER2, which is one of the most well-known markers in breast cancer. HER2 is also found in several other cancers, including gastric and ovarian, making it a high-value target if GT’s approach works.
Because the TriKE platform is modular, the science scales quickly. Once a delivery system is proven safe, it’s far easier to swap out targets and test new applications without having to rebuild everything from scratch. That’s the power of a true platform in biotech. The upfront work is heavy, but the upside comes from repeatability.
They’re not rushing. GT’s focus is on validating one success before expanding too fast. And for a company at this stage, that kind of discipline is often what separates real innovation from hype.
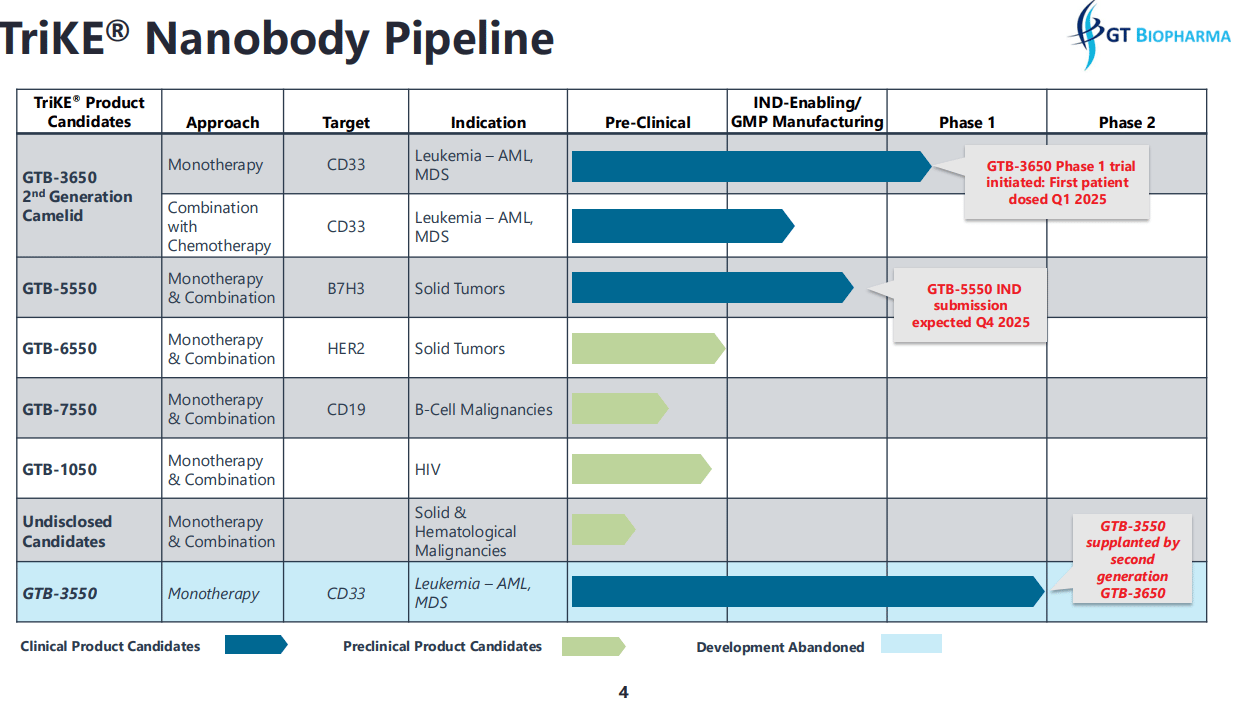
Financials and Strategic Setup
GT Biopharma is still early stage, but they’ve taken steps to extend their runway and reduce dilution risk.
As of the most recent update, the company reported around $17 million in cash. That gives them enough room to complete their ongoing trials and move additional TriKE programs closer to the clinic. They’ve also cut costs meaningfully over the past year and structured operations around clinical milestones, not bloated overhead.
In addition to the internal pipeline, GT has hinted at potential partnerships. Because TriKE is a plug-and-play system, it could be attractive to larger biotech and pharma players looking to add next-generation NK cell capabilities to their portfolios. If they strike the right deal, it could bring non-dilutive funding and external validation at the same time.
There’s still risk, of course. Biotech is unpredictable. But GT’s setup is lean, targeted, and positioned to make real progress over the next twelve to eighteen months.
Final Thought
GT Biopharma isn’t a household name. Most retail investors haven’t heard of TriKEs, and the market still tends to favor flashier biotech headlines. But this is one of those setups where the combination of platform potential, clinical data, and political momentum could create something interesting.
The company is still in the early innings, but they’re focused, funded, and showing real signs of progress. If the policy environment continues to shift in favor of faster approvals and experimental access, platforms like GT’s could benefit first.
This isn’t about chasing hype. It’s about understanding how science, strategy, and timing sometimes line up. And GT Biopharma is putting the pieces in place to be ready if and when that moment comes.
Cheers,
Matt Allen
COMMUNICATED DISCLAIMER — SPONSORED CONTENT: GT BIOPHARMA (NASDAQ: GTBP)
THIS DISCORD/TELEGRAM/WHATSAPP/FACEBOOK POST/WEBSITE/EMAIL NEWSLETTER/SMS TEXT IS A PUBLICATION OF MATT ALLEN. MATT ALLEN’S REPORTS AND RELEASES ARE PAID COMMERCIAL ADVERTISEMENTS FOR GENERAL INFORMATIONAL PURPOSES ONLY. MATT ALLEN IS ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION.
NEITHER MATT ALLEN NOR HIS OWNERS, OPERATORS, OR AFFILIATES HOLD ANY OWNERSHIP INTEREST, COMMON STOCK, OR DERIVATIVE SECURITIES OF GT BIOPHARMA (NASDAQ: GTBP) AT THE TIME OF THIS PUBLICATION. ALL OPINIONS, ANALYSES, AND STATEMENTS CONTAINED IN THIS PUBLICATION ARE ADVERTISING AND MARKETING MATERIALS AND SHOULD BE CONSIDERED AS SUCH.
INVESTING IN MICRO-CAP, LOW-FLOAT, OR EMERGING GROWTH STOCKS IS HIGHLY SPECULATIVE, CARRIES A HIGH DEGREE OF RISK, AND MAY RESULT IN THE LOSS OF YOUR ENTIRE INVESTMENT. YOU SHOULD NEVER INVEST FUNDS THAT YOU CANNOT AFFORD TO LOSE. THESE SECURITIES MAY BE SUBJECT TO EXTREME VOLATILITY, LIMITED LIQUIDITY, AND RAPID PRICE FLUCTUATIONS THAT CAN RESULT IN SUBSTANTIAL LOSSES.
MATT ALLEN IS NOT, AND DOES NOT CLAIM TO BE, A REGISTERED INVESTMENT ADVISOR, BROKER-DEALER, ANALYST, OR RESEARCH PROVIDER, AND DOES NOT PROVIDE PERSONALIZED INVESTMENT ADVICE. NEITHER MATT ALLEN NOR HIS OWNERS, OFFICERS, EMPLOYEES, OR AFFILIATES ARE REGISTERED WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (SEC), THE FINANCIAL INDUSTRY REGULATORY AUTHORITY (FINRA), OR ANY OTHER REGULATORY AUTHORITY. THIS PUBLICATION IS NOT AN OFFER TO BUY OR SELL, OR A SOLICITATION OF AN OFFER TO BUY OR SELL, ANY SECURITY OR FINANCIAL INSTRUMENT IN ANY JURISDICTION.
THE INFORMATION CONTAINED HEREIN IS BASED ON PUBLICLY AVAILABLE SOURCES, COMPANY PRESS RELEASES, FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION (INCLUDING BUT NOT LIMITED TO FORMS 10-K, 10-Q, 8-K, AND S-1), AND OTHER SOURCES BELIEVED TO BE RELIABLE. HOWEVER, MATT ALLEN MAKES NO REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, AS TO THE ACCURACY, COMPLETENESS, OR TIMELINESS OF SUCH INFORMATION. THIS PUBLICATION MAY CONTAIN FORWARD-LOOKING STATEMENTS THAT INVOLVE SIGNIFICANT RISKS AND UNCERTAINTIES. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THOSE STATED OR IMPLIED.
READERS ARE STRONGLY ENCOURAGED TO PERFORM THEIR OWN INDEPENDENT DUE DILIGENCE BEFORE MAKING ANY INVESTMENT DECISION, INCLUDING, BUT NOT LIMITED TO: REVIEWING ALL PUBLICLY AVAILABLE INFORMATION ABOUT THE COMPANY, CONSULTING WITH A LICENSED FINANCIAL ADVISOR, TAX PROFESSIONAL, OR LEGAL COUNSEL, AND CONSIDERING WHETHER SUCH AN INVESTMENT IS APPROPRIATE IN LIGHT OF THEIR INDIVIDUAL FINANCIAL CONDITION AND RISK TOLERANCE.
NEITHER THE PAYMENT NOR THE RECEIPT OF THIS PROMOTIONAL MATERIAL SHOULD BE CONSTRUED AS AN ENDORSEMENT OR RECOMMENDATION TO PURCHASE OR SELL ANY SECURITY. COMPENSATION PAID TO MATT ALLEN FOR THIS CAMPAIGN CREATES A MATERIAL CONFLICT OF INTEREST BECAUSE MATT ALLEN MAY HAVE A FINANCIAL INCENTIVE TO PROMOTE THE ISSUER. THIS NOTICE IS PROVIDED IN ACCORDANCE WITH SECTION 17(b) OF THE SECURITIES ACT OF 1933, WHICH REQUIRES THAT ANY PERSON WHO PROMOTES A SECURITY FOR COMPENSATION MUST FULLY DISCLOSE THE NATURE, SOURCE, AND AMOUNT OF SUCH COMPENSATION.
BY ACCESSING OR ENGAGING WITH THIS DISCORD/TELEGRAM/WHATSAPP/FACEBOOK POST/WEBSITE/EMAIL NEWSLETTER/SMS TEXT, YOU EXPRESSLY AGREE TO HOLD HARMLESS AND RELEASE MATT ALLEN, PENNY STOCK NEWS, LLC, AND THEIR RESPECTIVE OWNERS, OPERATORS, AFFILIATES, MEMBERS, EMPLOYEES, AND CONTRACTORS FROM ANY AND ALL LIABILITY FOR LOSS OR DAMAGE OF ANY KIND ARISING OUT OF OR RELATING TO THE USE OF THIS PUBLICATION. INVESTORS ARE REMINDED THAT PAST PERFORMANCE IS NOT INDICATIVE OF FUTURE RESULTS, AND THERE CAN BE NO GUARANTEE THAT THE COMPANIES DESCRIBED HEREIN WILL ACHIEVE THEIR GOALS, DELIVER RETURNS, OR MAINTAIN MARKET VALUE.
THIS COMMUNICATION IS STRICTLY FOR INFORMATIONAL AND ADVERTISING PURPOSES AND SHOULD BE TREATED ACCORDINGLY. MATT ALLEN HAS BEEN COMPENSATED ONE THOUSAND DOLLARS (USD) BY PENNY STOCK NEWS, LLC FOR THE DISSEMINATION OF THIS PROFILE ON BEHALF OF GT BIOPHARMA (NASDAQ: GTBP). THIS CAMPAIGN BEGINS ON 10/28/2025 AND ENDS ON 10/28/2025.
Forward-Looking Statements
Certain information provided in this communication may contain “forward-looking statements,” which include “future-oriented financial information” and “financial outlook” under applicable securities laws. These forward-looking statements are based on current beliefs and expectations of the Company’s management regarding its business, financial performance, strategic plans, and market conditions. These statements may include, but are not limited to, projections on the Company’s financial performance, the expected use of proceeds from securities offerings, development of business and joint ventures, execution of growth strategies (including potential mergers and acquisitions), availability of financing, the completion of ongoing projects, renewal of material agreements, and future capital and liquidity requirements.
Forward-looking statements are intended to provide investors with management’s views about potential future developments, but they are not guarantees of future performance. These statements involve risks and uncertainties that may cause actual results to differ significantly from projections or expectations. Investors should not place undue reliance on these forward-looking statements, as actual results may vary due to factors beyond the Company’s control.
While the Publisher believes the assumptions behind the forward-looking statements are reasonable, no assurance can be given that these projections will prove accurate. The Publisher does not undertake any obligation to update or revise forward-looking statements unless required by applicable securities laws. The reader is strongly advised to make their own investment decisions and should not rely solely on these forward-looking statements when making investment choices.
Disclaimer for BeanWealth
BeanWealth is a publisher of financial education and information. We are not an investment advisor and do not provide personalized investment advice or recommendations tailored to any individual's financial situation. The content provided through our website, newsletters, and any other materials is for educational purposes only and should not be construed as financial or investment advice.
All information is provided “as is,” without warranty of any kind. BeanWealth makes no representations or guarantees regarding the accuracy, completeness, or timeliness of the information presented. The opinions and views expressed in our content are those of the author(s) and do not necessarily reflect the views of BeanWealth, its partners, or its affiliates.
Investors should perform their own due diligence and consult with a professional financial advisor before making any investment decisions. None of the information provided herein constitutes a solicitation to buy or sell any securities or financial instruments. Any projections or forecasts mentioned are speculative and subject to risks and uncertainties that could cause actual outcomes to differ.
BeanWealth, its employees, and affiliates may hold positions (long or short) in the securities or companies mentioned, and these positions may change without notice. No guarantees are made regarding the continuation of these positions.
Forward-looking statements, estimates, or forecasts provided are inherently uncertain and based on assumptions that may not occur. Other unforeseen factors may arise that could materially affect the actual outcomes or performance of the securities discussed. BeanWealth has no obligation to update or correct any information after the date of publication.
BeanWealth disclaims any liability for losses or damages, whether direct or indirect, resulting from the use of the information provided. By accessing or using any BeanWealth content, you agree to this disclaimer and our terms of service.
Unauthorized distribution, reproduction, or sharing of this content is strictly prohibited and subject to legal action.